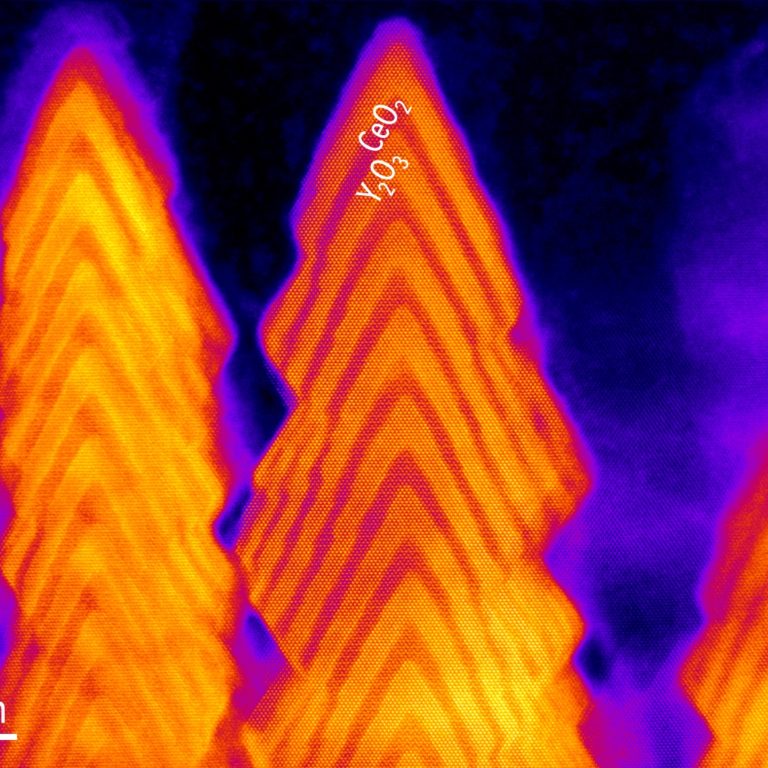
Colossal oxygen vacancy formation at a fluorite-bixbyite interface
Ho Nyung Lee | Posted on |
Researchers discovered a novel synthesis method to grow nanobrush architectures with interfaces designed to form high-density oxygen vacancies. The rational design of interfacial oxide architectures to create, control, and transport oxygen vacancies could be applied to various energy and information technologies, such as solid oxide fuel cells and memristive devices.
Pulsed laser deposition (PLD) was used to grow a nanobrush architecture under conditions of surplus feedstock, forming atomically well-defined (111) interfaces within CeO2–Y2O3 (fluorite–bixbyite) superlattices. Analysis of local structure and chemical composition, with scanning transmission electron microscopy, atom probe tomography, and neutron scattering as well as theoretical calculations, suggested that more than 10% of oxygen atoms could be removed without degrading the crystalline quality. The underlying mechanism originates from interfacial charge modulation when the fluorite CeO2 (Ce4+) and the bixbyite Y2O3 (Y3+) create a (111) interface. The precision growth technique to synthesize nanobrush architectures developed here offers many advantages over 2D thin films, including much greater surface areas and a larger number of interfaces than exists in planar films—useful for many important technological applications.
D. Lee, X. Gao, L. Sun, Y. Jee, J. Poplawsky, T. O. Farmer, L. Fan, E.-J. Guo, Q. Lu, W. T. Heller, Y. Choi, D. Haskel, M. R. Fitzsimmons, M. F. Chisholm, K. Huang, B. Yildiz, and H. N. Lee, “Colossal oxygen vacancy formation at a fluorite-bixbyite interface,” Nature Commun. 11, 1371 (2020).